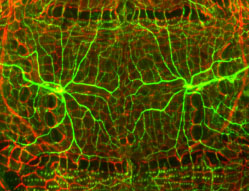
For an organism to survive, it is critical to assess its surroundings and avoid any potentially damaging stimuli. Nociception, sensation of noxious stimuli, is a key function of the sensory nervous system and it is an evolutionarily conserved protection mechanism. The research interests we pursue in the lab is the molecular and genetic underpinnings of neuroplasticity of pain sensation. Using Drosophila as our genetically tractable model system, we ask fundamental questions – how pain sensation can be modulated? Can disease conditions alter pain sensitivity? What cellular changes accompany in the sensory neurons when pain sensitivity is altered? We address these questions on the basis of disciplines in Genetics, Biochemistry, Cell and Molecular Biology, and we use the expansive genetic and imaging tools available in Drosophila model system.
Pain Modulation by Neuropeptide Signaling
We model injury-induced nociceptive sensitization in flies by exposing them to UV radiation and then testing their pain responses. Using this model, I discovered that Drosophila substance P (sub P) signaling modulates thermal pain sensitization, and its activation is required in the nociceptive sensory neurons (Im et al., eLife, 2015).Even though sub P signaling is known for many years to function in pain in mammals, many clinical trials using sub P inhibitors have so far produced low efficacy in reducing pain. This impediment is partly due to the lack of a precise understanding of how the sub P pathway modulates nociception. My work filled this gap in knowledge by providing genetic mechanisms that Drosophila sub P functions upstream of hedgehog (Hh) signaling during pain sensitization, revealing a novel genetic interaction in pain biology. We aim to further dissect the molecular mechanisms of sub P function, and (1) address the activation mechanisms of sub P signaling following injury, and (2) dissect genetically and biochemically how sub P and Hh signaling interact.
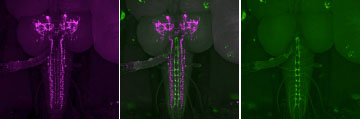
(Im et al., eLife, 2015)
Insulin Signaling and Diabetes-induced Pain Hypersensitivity
Taking advantage of the Drosophila genetic system, I screened through Drosophila kinases and found a number of them involved in pain modulation. Among these kinases, the Insulin receptor is required in the sensory neurons to control the duration of pain hypersensitivity. Loss of Insulin signaling, either due to genetic manipulation or diabetic conditions, triggers persistent pain hypersensitivity, which lasts longer than the normal transient pain hypersensitivity following injury (Im et al., DMM, 2018). This is an interesting discovery highlighting an underappreciated role of Insulin signaling in neuroplasticity.Using various approaches encompassing genetics, biochemistry, molecular and cellular biology, and imaging tools, my lab will study (1) how Insulin signaling is regulated in the nociceptive sensory neurons, (2) whether it is involved in other modalities of pain, and (3) what might be therapeutic targets to ameliorate the pain syndromes of diabetic patients.
Finally, diverse physiological processes/conditions can affect pain sensitivity. Future directions we are interested include investigating the interaction between pain and other physiological processes, such as learning and memory, immunity, and sleep quality. We welcome collaboration ideas and will be excited by the opportunity to contribute to the bigger and general field of biological and biomedical research.
Interplay between pain and sleep
Chronic pain patients often report disrupted sleep patterns, while conversely, impaired sleep cycles can exacerbate current pain conditions. Clinically, sleep regulation has been suggested as a life style treatment option, indicating that there exists mutually beneficial relationship between the quality of sleep and pain sensitivity. How sleep and pain are related? What genetic or cellular mechanisms are involved in this interaction? To understand the relationship between sleep and pain, we will modulate and monitor sleep patterns and pain sensitivity in Drosophila melanogaster. Merging research experience in circadian rhythms /sleep and in nociception, this is a new and specialized research program for the Im lab research.